Introduce the Valganciclovir Hydrochloride in detail
2023-10-17
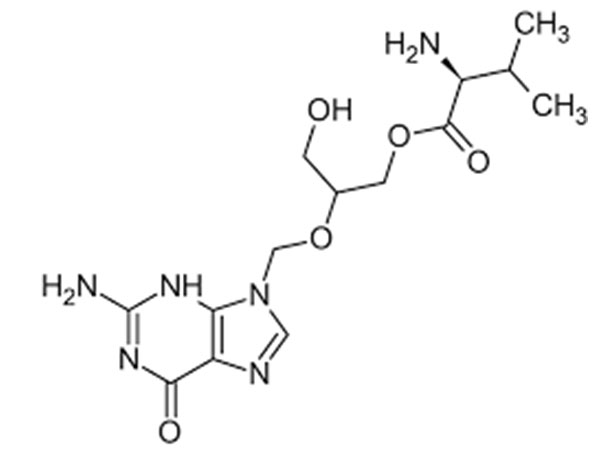
Valganciclovir hydrochloride is an antiviral medication that is primarily used for the treatment and prevention of cytomegalovirus (CMV) infections. It is an oral prodrug of ganciclovir, which means that it is converted into ganciclovir in the body and exhibits similar antiviral properties. Valganciclovir hydrochloride is available in tablet form and is prescribed under various brand names.
Here are the key details about valganciclovir hydrochloride:
Mechanism of Action:
Valganciclovir hydrochloride works by inhibiting the replication of CMV, a member of the herpesvirus family. After being converted into ganciclovir, it is phosphorylated by viral and cellular kinases and incorporated into the viral DNA, leading to chain termination and inhibition of viral replication.
Indications:
1. Prevention of CMV Disease: Valganciclovir hydrochloride is commonly used to prevent CMV disease in immunocompromised individuals, such as those undergoing solid organ transplantation (kidney, heart, liver) or hematopoietic stem cell transplantation.
2. Treatment of CMV Retinitis: It is also approved for the treatment of CMV retinitis, a viral infection affecting the retina, in patients with acquired immunodeficiency syndrome (AIDS).
Dosage and Administration:
The dosage of valganciclovir hydrochloride depends on the indication, patient's weight, renal function, and other factors. It is typically administered orally as tablets and should be taken with food to enhance absorption. The treatment duration may vary depending on the individual's condition and response to therapy.
Adverse Effects:
Common side effects of valganciclovir hydrochloride may include diarrhea, nausea, vomiting, headache, fatigue, and neutropenia (reduced white blood cell count). It may also cause more serious adverse effects, such as bone marrow suppression, anemia, thrombocytopenia, and liver toxicity. Regular monitoring of blood counts and liver function is necessary during treatment.
Precautions and Contraindications:
1. Pregnancy and Breastfeeding: Valganciclovir hydrochloride is classified as a Category C medication in pregnancy, meaning that it may cause harm to the fetus. It should be used during pregnancy only if the potential benefits outweigh the risks. It is generally not recommended during breastfeeding.
2. Renal Impairment: Dose adjustment is required in patients with impaired renal function. Close monitoring of renal function is necessary, and dosage modification may be needed based on the creatinine clearance level.
3. Hematologic Disorders: Valganciclovir hydrochloride can cause bone marrow suppression, leading to decreased blood cell counts. It should be used with caution in individuals with pre-existing hematologic disorders.
4. Drug Interactions: Valganciclovir hydrochloride may interact with other medications, including myelosuppressive drugs, immunosuppressive agents, and drugs eliminated through the kidneys. It is important to inform the healthcare provider about all concomitant medications.
It is essential to follow the prescribed dosage and duration of treatment as directed by a healthcare professional when using valganciclovir hydrochloride. Regular monitoring, including blood tests and ophthalmologic examinations, may be necessary to assess treatment efficacy and detect potential side effects.